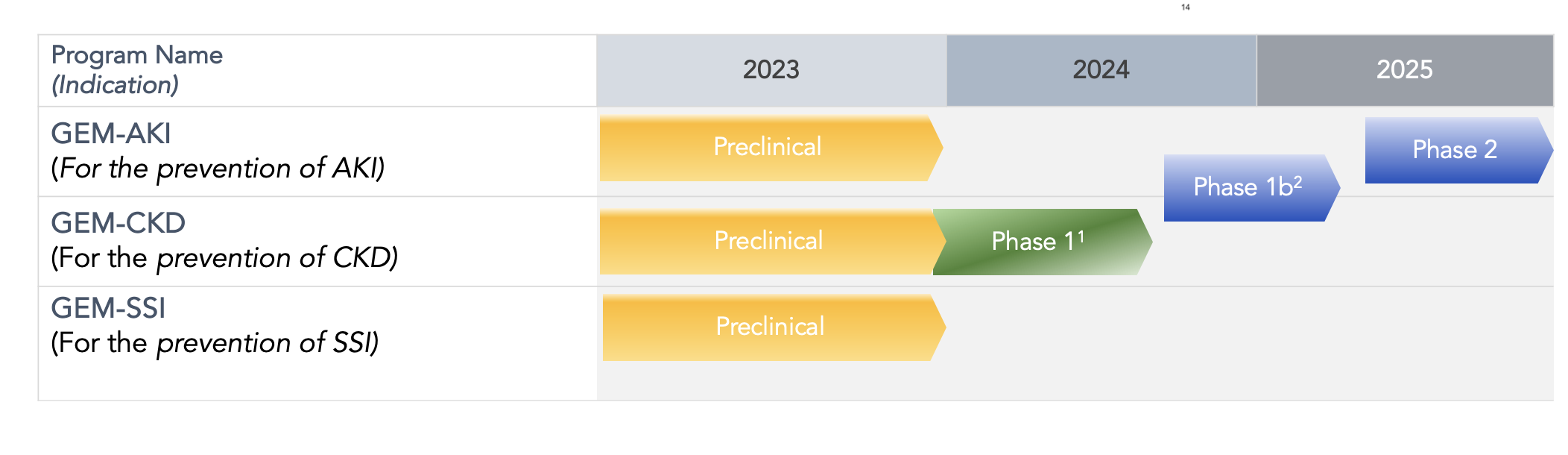
PIPELINE
About Gemini
Gemini is a proprietary formulation of phosphorylated hexaacyl disaccharide (PHAD®) for systemic administration. It is being developed for multiple indications including as a pretreatment to prevent or reduce the severity and duration of post-surgical infection (GEMINI-SSI program), as pretreatment to prevent or reduce the severity and duration of acute kidney injury (GEMINI-AKI program). In addition, Gemini may be a treatment to stop or slow the progression of chronic kidney disease (GEMINI-CKD program). Revelation believes Gemini works through trained immunity, which redirects and attenuates the innate immune response to external stress (infection, trauma, etc.). Revelation has conducted multiple preclinical studies demonstrating the therapeutic potential of Gemini in the target indications.
GEM-AKI
GEM-AKI is being developed as a potential therapy for the treatment of acute kidney injury (AKI). We believe redirection of the innate immune response with GEM-AKI from a pro-inflammatory state to an anti-inflammatory (protective) state may rebalance the innate immune response to slow down or halt the progressive destruction and scarring of organ tissue, allowing the healing process to take place. Preclinical studies conducted by our scientific advisors at Vanderbilt have shown that systemic pretreatment with PHAD results in significant protection from AKI and On February 7, 2023, we announced results from a preclinical study where administration of Gemini showed the protective effect of IL-10 and NGAL upregulation on the formation of kidney scar tissue in a validated preclinical model of acute and chronic kidney injury.
GEM-CKD
GEM-CKD is being developed as a potential therapy for the treatment of chronic kidney disease (CKD). We believe redirection of the innate immune response with REVTx-300 from a pro-inflammatory state to an anti-inflammatory (protective) state may rebalance the innate immune response to slow down or halt the progressive destruction and scarring of organ tissue, allowing the healing process to take place.
GEM-SSI
GEM-SSI is being developed, through a license agreement with Vanderbilt University, as a potential therapy for the prevention or treatment of healthcare-associated bacterial infection including post-surgical infection, post-burn infection, urinary tract infection (e.g. as a result of hospital-based or outpatient catheterization), sepsis, antibiotic-resistant infection, etc. We hypothesize stimulation of Toll like receptor-4 (TLR-4) will selectively prime the body’s immune system to be able to better respond to subsequent pathogen exposure.
Multiple preclinical studies conducted by our scientific advisors at Vanderbilt have shown that systemic pretreatment with PHAD results in significantly increased immune response leading to significantly reduced duration and severity of infection following bacterial challenge with either gram-positive or gram-negative bacteria.
Additional studies conducted by Revelation have shown that Gemini rapidly and significantly mobilizes white blood cells and key cytokines of activity in a dose dependent manner in healthy animal studies. Revelation plans to demonstrate this same rapid mobilization as part of our Phase 1 healthy volunteer clinical study. Demonstration of this phenomenon will be a good indicator of future activity in patients.